Endometriosis is a still little known gynecological disease. It causes very strong pain in women. In the United States, the Food and Drug Administration (FDA) has just approved a treatment intended to relieve them. It’s a first.

One in ten women of childbearing age suffers from endometriosis, a disease that is still too taboo and too little known in France, even if personalities, such as Enora Malagré, testified in the media. Recently in the United States, the Food and Drug Administration (FDA, the equivalent of the National Medicines Safety Agency in France) has approved the marketing of a drug to relieve pain associated with the disease. A first in more than ten years.
Decreased estrogen production
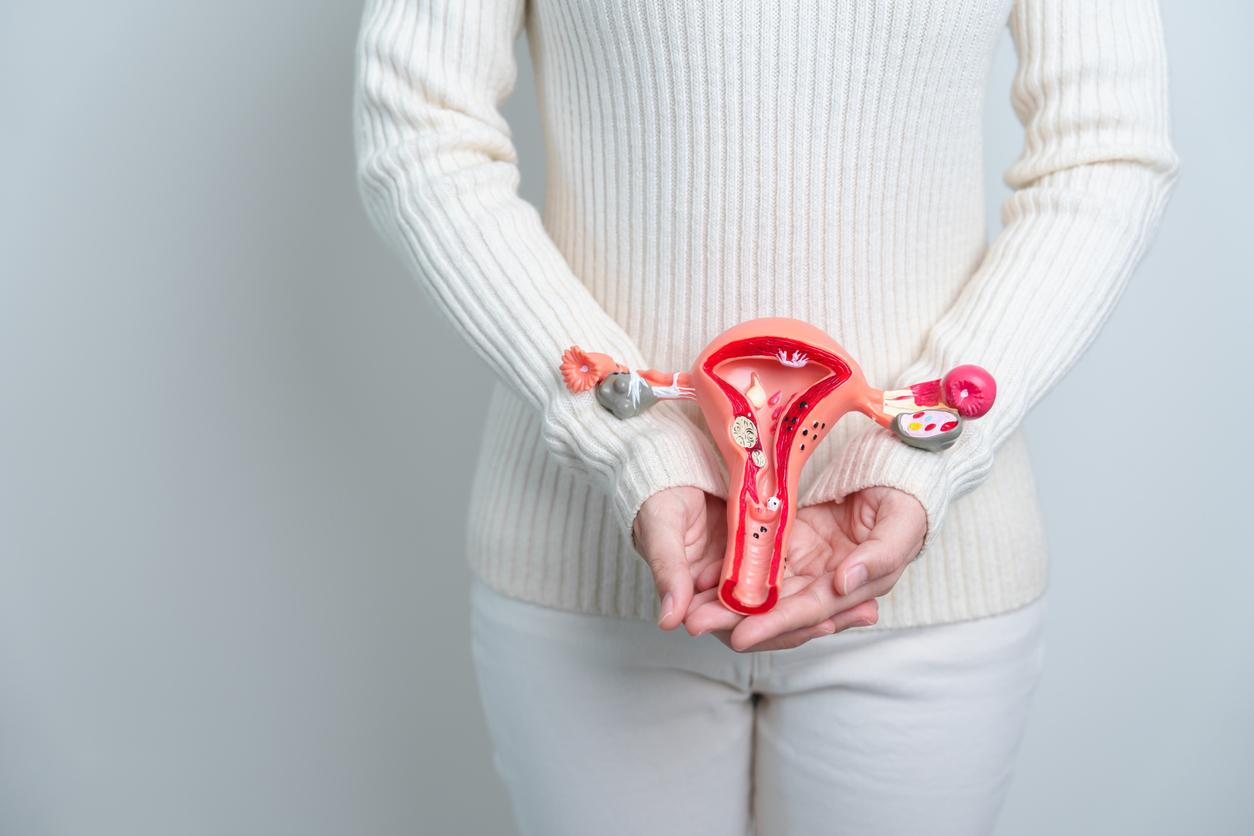
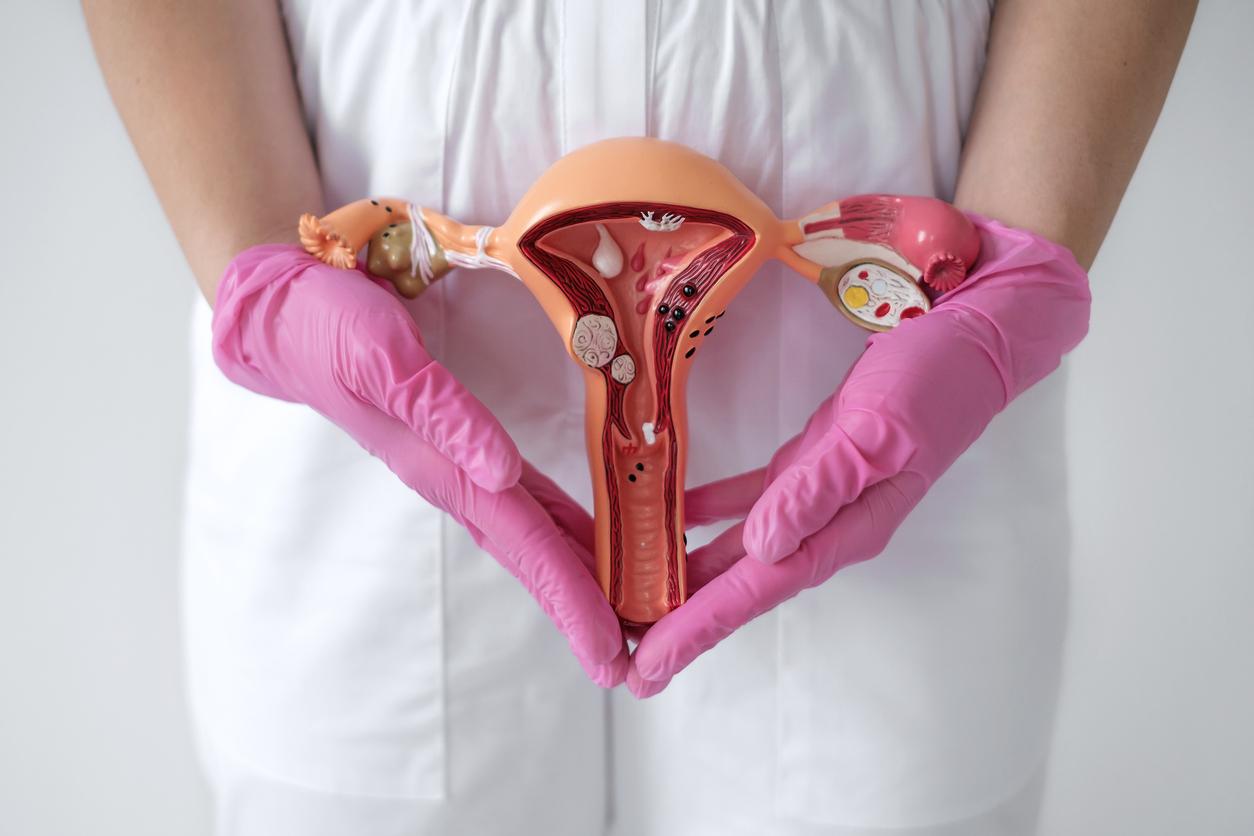
Endometriosis is characterized by the presence of uterine cells outside the uterus. These cells react to estrogen, the female hormones, during menstruation, which causes severe pain. Women who have it also suffer during sex and are at risk of infertility.
About a year ago, the New England Journal of Medicine published the results of the first clinical trials. Baptized Orilissa, the treatment tested reduces menstrual and pelvic pain (in the lower abdomen and genital area). Composed of the active ingredient elagolix, it allows to decrease the production of estrogen.
Side effects
But taking Orilissa is not without consequences. Among the side effects, the decrease in bone density and therefore the risk of osteoporosis or the increased risk of miscarriages. According to Yasmine Candau, president of the French association for the fight against endometriosis EndoFrance questioned by LCI, this treatment is not an innovation either. It would be an alternative to an already existing treatment, but which works by injection.

.