According to a new assessment by the French National Authority for Health, the actual benefit of Pradaxa® would be less than that of Eliquis® and Xarelto®. Boehringer Ingelheim, manufacturer of Pradaxa®, disputes.
The Transparency Commission of the HAS (Haute Autorité de Santé) has reassessed three new oral anticoagulants (NACO). According to his analysis, these newer anticoagulants show varying efficacy, particularly when prescribed for the prevention of stroke and embolism, in patients with non-valvular atrial fibrillation.
Apixaban (Eliquis®), dabigatran (Pradaxa®) and rivaroxaban (Xarelto®) entered the market in 2008, explains HAS in a press release. During their initial assessment, the HAS had considered that they were all rendering an important medical service. Seized by the Ministry of Health, the HAS had to rule again.
A difference in benefits
The Transparency Committee’s analysis led to a differentiation between drugs. Thus, the actual benefit is considered “significant” for Eliquis® and Xarelto®. On the other hand, it is only “moderate” for Pradaxa®.
The HAS is in favor of maintaining the three molecules on the list of reimbursable specialties. However, moderate actual benefit most often leads to a reassessment of the reimbursement rate, which would drop for Pradaxa to 30%, instead of 65%.
First-line antivitamin K
More generally, the HAS was invited to comment on the place of these drugs in the strategy of prevention of strokes and embolisms. However, the Commission considers that, in the current state of knowledge, they should be prescribed as a second-line treatment, in particular because of the current absence of an antidote; molecules are under development. Antivitamin K (AVK) should be prescribed as a first-line treatment.
According to the HAS, the NACOs are to be reserved for two situations. On the one hand, they are intended for patients on AVK for whom the maintenance of the desired INR (International Normalized Ratio) in the target zone is not guaranteed, despite correct compliance. On the other hand, they are to be prescribed to patients for whom VKA are contraindicated or poorly tolerated.
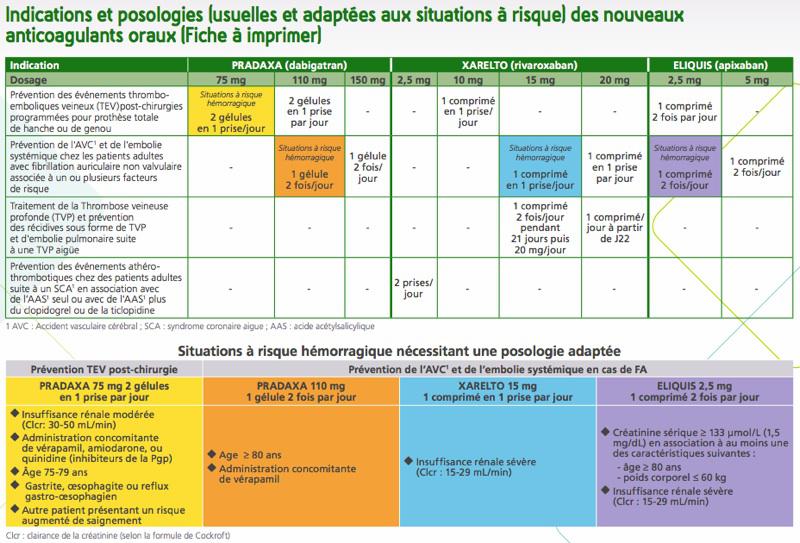
“The HAS will update and distribute to the attention of prescribers a card on the proper use of these drugs”, conclude the authors of the press release. For its part, the National Medicines Safety Agency (ANSM) has developed, in its latest bulletin “Vigilances”, tables intended for health professionals to help them prescribe these molecules.
The lab reacts
The reaction of Boehringer Ingelheim, manufacturer of Pradaxa®, was not long in coming. The laboratory “neither understands nor shares the unfavorable assessment of the Commission”, one can read in a press release. The laboratory fulminates: “The experience of Pradaxa® represents more than 3.5 million patient-years in all its authorized indications throughout the world since it was made available to prescribers more than 7 years ago”. Boehringer Ingelheim says he is “confident in the effectiveness” of his drug.
The pharmaceutical company also underlines to reserve the right “to use all legal remedies to appeal the consequences of this opinion concerning the reimbursement” of the drug. To be continued…
.

















