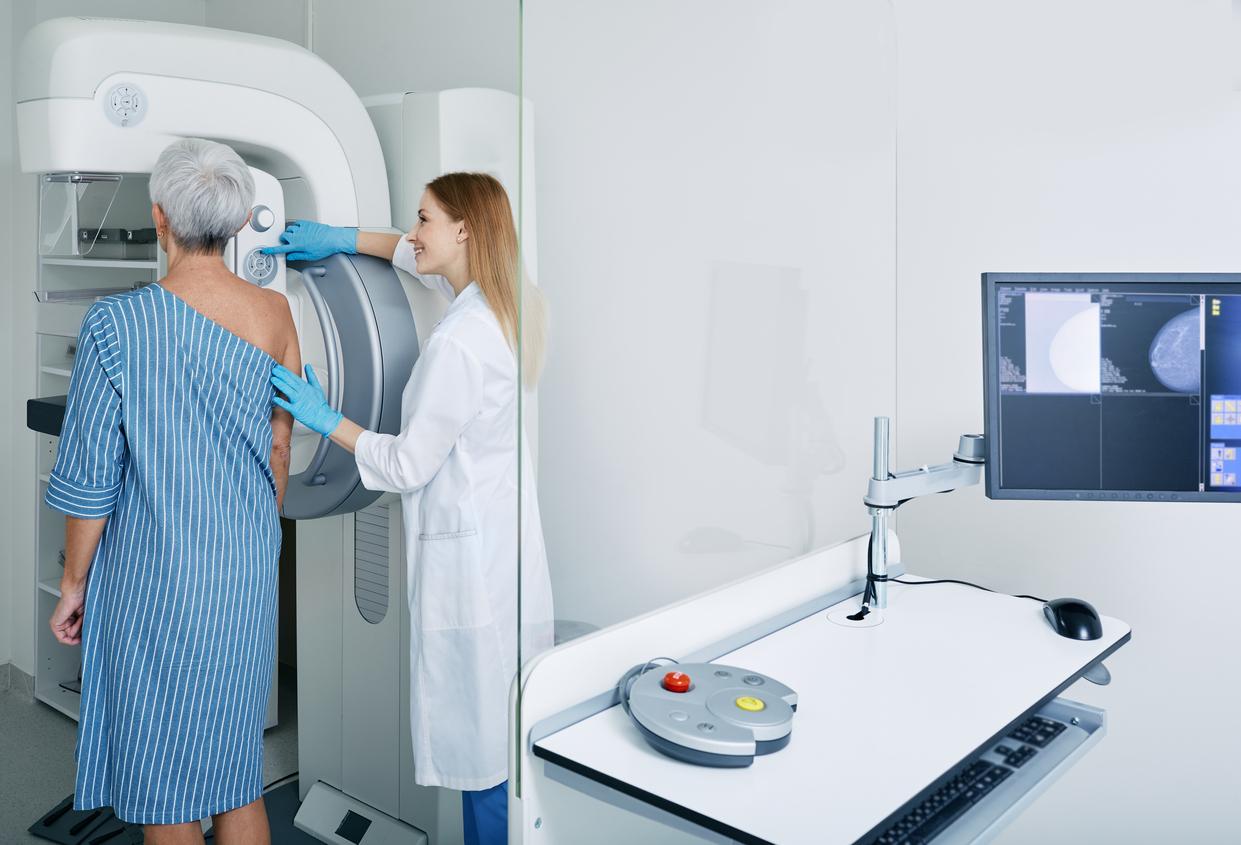
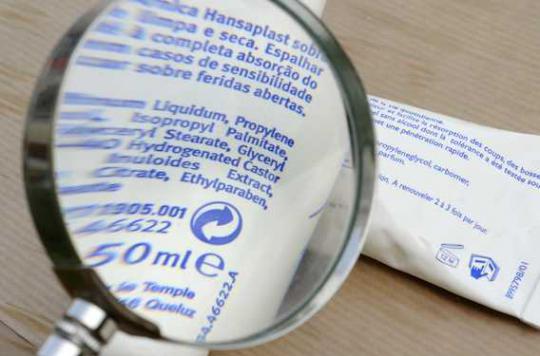
At the end of 2012, the Medicines Agency advised against the use of baby wipes containing phenoxyethanol. A survey of 60 million consumers reveals that they have not disappeared from the shelves.

Cosmetics, detergents, or even food, all product families contain undesirable ingredients whose repeated contact with humans could have a real impact on their health. This is the worrying conclusion of a major survey published a few days ago in the magazine 60 Million Consumers. For example, Coca-Cola, Carambar, and other Burn energy drinks contain a cancer-causing dye called E150D. Moreover, well-known cosmetic products such as Colgate or Sanogyl toothpastes have in their composition tricolsan, a bacterial agent suspected of promoting resistance to antibiotics. But even more worrying, the National Institute of Consumption also points the finger at the dangerosty of certain baby products, this is the case for example of cleaning wipes containing phenoxyethanol.
Present in dermatological products, but also in many baby wipes, phenoxyethanol is part of the family of glycol ethers. These chemicals exhibit remarkable properties. They are miscible in water and fats and also allow the mixing of immiscible products. They are not very volatile and odorous. In the 1970s, glycol ethers replaced certain flammable and neurotoxic solvents and invaded our daily life.
This is the reason why these substances have been under surveillance since the 2000s. Toxic effects for reproduction have indeed been demonstrated in animals. So, even if for the moment no such thing has been reported in humans, the Drug Safety Agency has already issued recommendations for its use in the past.
Indeed, following a Evaluation that it carried out last year, the Ansm advises since November 2012 “as a precaution” not to use wipes intended for the seat for babies containing phenoxyethanol (code name of EGphE). “There are no elements of concern but just a question for lack of sufficient data on the degree of absorption by the skin of babies of this glycol ether but which can be toxic for the blood and the liver”, according to Brigitte Heuls, director of therapeutic and cosmetic medical devices at the Agency.
In its conclusions, the Ansm also recommended a restriction of phenoxyethanol to 0.4% in all other cosmetic products intended for children against the 1% currently.
But obviously, these recommendations issued by the Ansm did not appeal to the manufacturers since, as shown by the survey carried out by the monthly 60 million consumers, “pheonoxyethanol baby wipes from big names in the sector continue to grow. to fill in the shelves ”. However, things may well change in the future as the Agency has forwarded its report to the European Commission “for a Community assessment.” “
.