Tested for 2 years, the first wireless heart pacemakers are showing promising results. This new technology heralds a turning point in the management of patients suffering from arrhythmias.
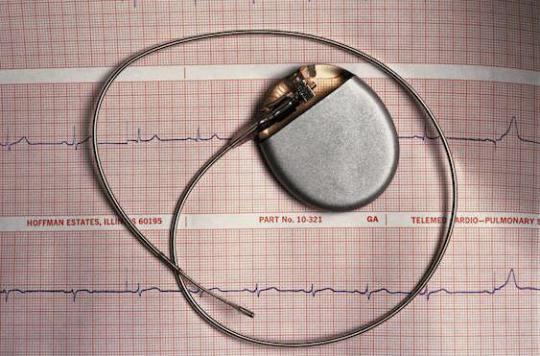
Since the first models, developed in the 1950s, the implantation of pacemakers has become a routine procedure. Today, these devices are small, light and their battery gives them an autonomy of 7 to 15 years. However, a pitfall persists: the leads that connect the box implanted under the skin to the tip of the cardiac ventricle. These can be a source of infections, or simply be damaged. To overcome these constraints, wireless pacemakers have been developed. The results of tests carried out in recent months, published in the New England Journal of Medicine were presented at the annual congress of the European Society of Cardiology, which took place in London from August 29 to September 2. Dr Jacques Gauthier, President of the College of French Cardiologists, provides an update with Dr Jean-François Lemoine on these new devices.
A minimally invasive implantation
Without probes, the Nanostim stimulator, developed by the company St Jude Medical, is implanted directly in the heart. The operation, which takes half an hour, is only minimally invasive: the pacemaker, which is in the form of a small 4-centimeter rod, is introduced into the right ventricle via the femoral artery. It is then attached to the bottom of the heart chamber.
Since the first implantation, carried out at the end of 2013, by cardiologists from Grenoble, a little more than 500 patients have been fitted with a Nanostim pacemaker. Data collected during an individual 6-month follow-up showed that the device was effective in 93% of cases. However, incidents have been identified in more than 6% of implanted patients, mostly a displacement of the pacemaker or cardiac perforation. “These initial results are encouraging,” comments Jacques Gauthier. However, we must remain cautious: the follow-up is still short and above all the 1000 patients planned in the clinical trial have not yet all been implanted. “
A technology reserved for certain cases
If ongoing clinical trials evaluating the safety and efficacy of Nanostim, as well as other models (e.g. Micra, produced by Medtronic), prove successful, wireless pacemakers could appear on the market in the future. coming years. But this technology should remain, at least initially, reserved for a small fringe of patients. “” Vv “x
As for the question of the withdrawal of these miniature models, in the event of damage or of battery change, for Jacques Gauthier it is “more likely that a new pacemaker is fitted while leaving the previous one in place”. A procedure which would not pose a problem of congestion, “the right ventricle is very compliant”, smiles the cardiologist.
A technology reserved for certain cases
If ongoing clinical trials evaluating the safety and efficacy of Nanostim, as well as other models (e.g. Micra, produced by Medtronic), prove successful, wireless pacemakers could appear on the market in the future. coming years. But this technology should remain, at least initially, reserved for a small fringe of patients. “These miniature pacemakers can only replace the simplest pacemakers, whose function is to trigger stimulation at the tip of the heart,” warns Jacques Gauthier. This represents 10 to 15% of patients who suffer from arrhythmias. “
Find the full debate on wireless pacemakers in our Health TV section:

.