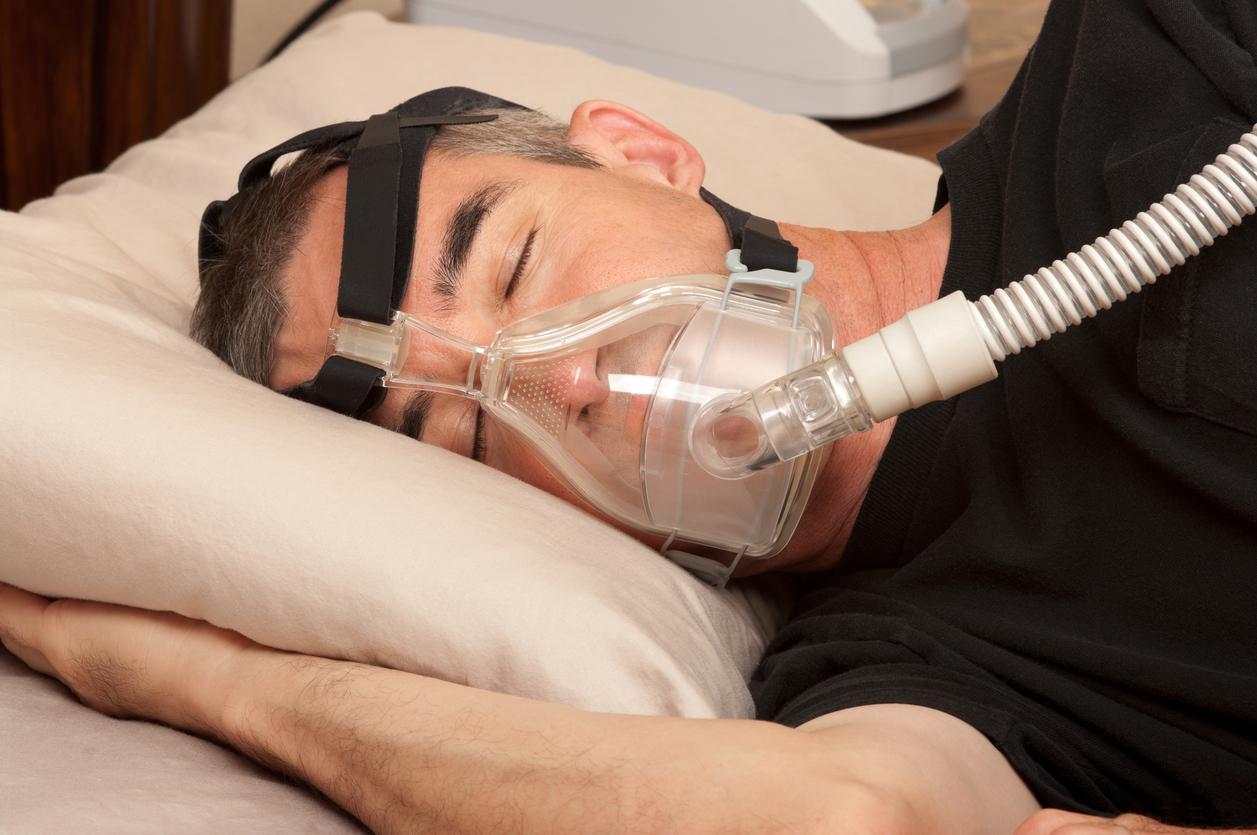
Because it is taking too long to recall 370,000 respiratory devices, deemed harmful to health, the industrialist Philips will be the subject of collective legal action by users in the coming days. For its part, the Medicines Agency has taken a health policy decision to speed up the replacement of defective devices.
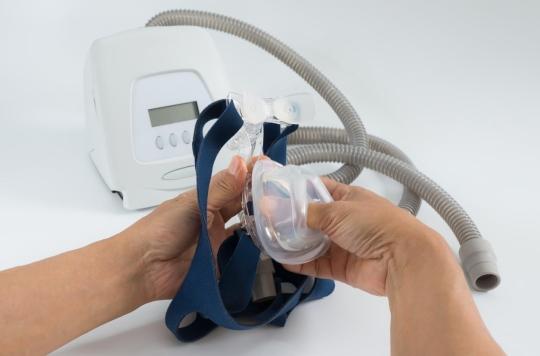
- Sleep apnea is characterized by involuntary interruptions in breathing during sleep. These respiratory arrests last more than 10 seconds and sometimes reach more than 30 seconds. They can repeat themselves more than 30 times per hour of sleep.
- Breathing pauses cause a lack of oxygen resulting in a short awakening. Breathing then resumes to stop again a little later. Sleep is therefore of poor quality.
A situation that is not “more acceptable”. Here is how Caroline Semaille, deputy director of the National Agency for the Safety of Medicines and Health Products (ANSM) described the slowness with which the manufacturer Philips is recalling its defective respirators. Deemed harmful to health, these respirators could even prove to be carcinogenic due to the particles of sound-absorbing foam when inhaled or ingested by the user.
In France, the recall procedure concerns the following devices, manufactured between November 2009 and April 2021:
– REMstar Pro, Auto, Expert (DreamStation, PR1 / SystemOne, Q-series)
– BiPAP Auto
– DreamStation Go
ANSM launches a health policy decision
Marketed for people suffering from sleep apnea, these positive pressure devices provide airflow in the airways. They are connected to a mask that must be worn on the face, and the air is routed through a flexible hose.
Problem: since June 14, Philips has announced on his website that it was recalling several of its models due to health risks associated with their use. “Potential risks include exposure to particulate matter from degraded foam, which may for example be caused by the use of unapproved cleaning/disinfection methods such as ozone, and exposure to volatile organic compound (VOC) emissions from moss. In some areas, high heat and high humidity environments can also contribute to the degradation of this moss.”, details Philips on its website. In total, 370,000 devices are affected by this recall procedure.
However, for eight months, only 7% of devices have been taken back, the ANSM informed during a press conference held on Tuesday February 8, which makes it say that “Philips does not respect its commitments”.
To accelerate the replacement of these dangerous devices, the ANSM therefore announced on Friday February 11 initiate a health policy decision. This means that Philips exposes itself to criminal proceedings if it does not respect the timetable imposed by the ANSM, namely the replacement of three quarters of the defective devices by the end of June and all of them by the end of the year. She also wants the manufacturer to launch “an epidemiological study to assess the risk of cancer potentially induced by exposure to the ventilation equipment concerned, the preliminary results of which must be sent to the ANSM within one year at the latest”.
A class action lawsuit against Philips
For their part, users of the devices against sleep apnea have announced their intention to launch collective legal proceedings against Philips. Led by Me Christophe Lèguevaques, lawyer at the Paris Bar, this procedure is made public on the MyLeo collective action platform. “All users of these devices can participate”said the lawyer to RMC. According to him, “two procedures are possible for the plaintiffs: a collective action to obtain compensation in the event of moral damage of anxiety, but also a penal action to know the truth and punish the culprits”.
Users have until April 11 to submit documents for their file on MyLeo. “For civil proceedings, you need at least 1,000 participants, but this is a number that we think we can reach quite easily because there are potentially 370,000 people concerned in France. For the criminal procedure, it only takes two plaintiffs, and we already have them, sick people who want to know what happened”added Me Lèguevaques.

.