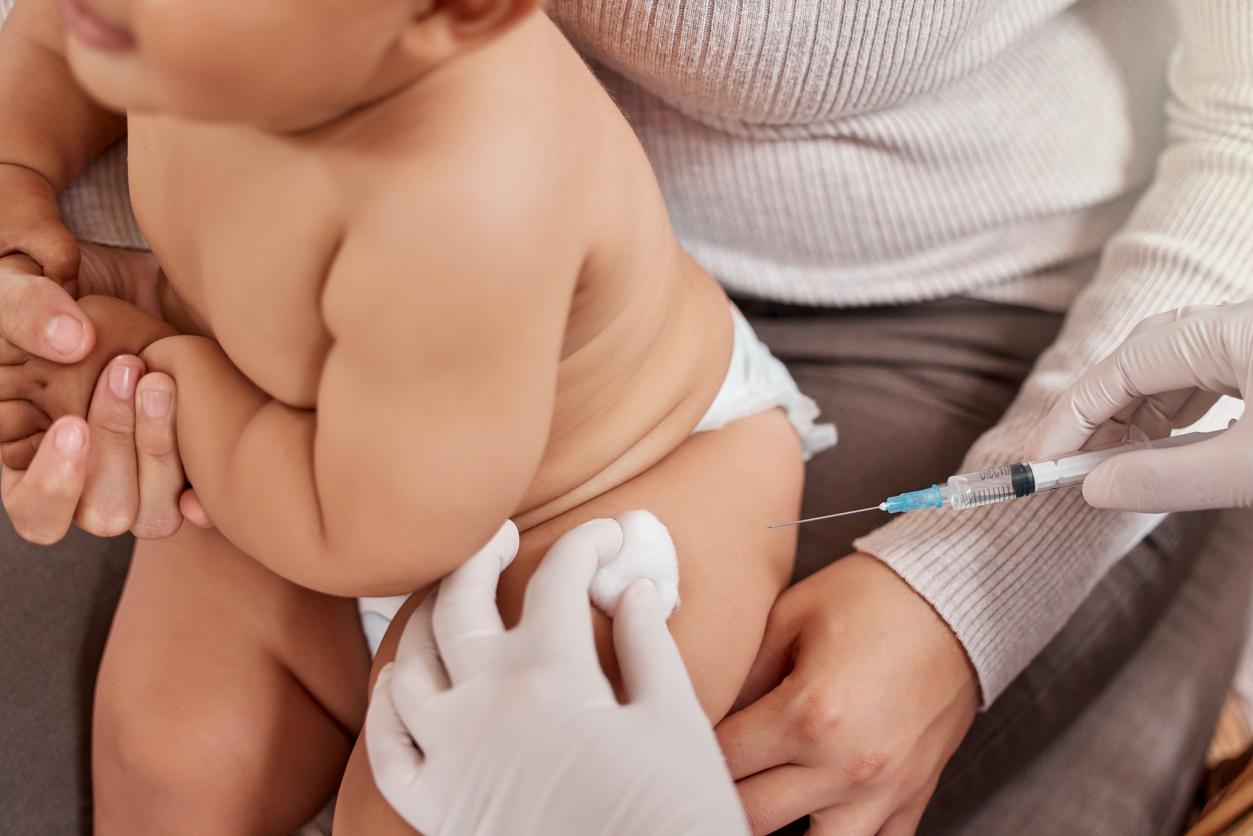
The government has opened the possibility for pharmacies to carry out vaccination against the Mpox virus. However, pharmacies must meet several conditions to benefit from this authorization.

- In France, only five pharmacies could carry out vaccination against Mpox as part of an experiment launched in August 2022.
- All French pharmacies can now apply to vaccinate against the Mpox virus, but they will have to respect several conditions to obtain this right.
- Vaccination against the Mpox virus only concerns certain categories of the population.
On October 22, a first case of infection with the new variant of the Mpox virus, formerly called monkeypox, was detected in Germany. “On October 18, 2024, an Mpox infection with the new clade 1b, acquired abroad, was detected in Germany.announced the Robert Koch Health Monitoring Institute (RKI), located in Berlin. According to this organization, “the risk to the health of the population Germany” was “weak”. This viral disease is a zoonosis, in other words it is transmitted from animals to humans as well as between humans. Close contact with an infected person can therefore promote the spread of this pathology.
Mpox vaccine: all pharmacies can carry it under certain conditions
In France, health authorities recommend vaccination against this virus for certain populations. In a decree published in Official Journal However, on October 23, the government decided to strengthen the vaccination system by offering the possibility to all pharmacies to offer serum against Mpox.
Since August 2022, an experiment had been set up by the government: only five pharmacies offered vaccination against the Mpox virus. With the publication of the decree, all pharmacies can now ask the Regional Health Agencies (ARS) to join the vaccination circuit. However, these health authorities may refuse this request.
To obtain vaccination authorization, pharmacies must meet several conditions such as:
- present an interest in covering the territorial coverage of the vaccination offer;
- present geographic proximity to the populations targeted by vaccination;
- be able to support patients in prevention of the monkeypox virus and, more broadly, sexual health;
- preferably be in geographical proximity to a pharmacy for indoor use allowing the supply of thawed vaccine doses and medical devices relating to this vaccination by such a pharmacy;
- have a daily vaccination capacity making this offer relevant;
- justify an appointment-making system that is efficient and visible to the greatest number of patients;
- enter the doses administered in the tool provided to them.

Who is affected by vaccination against the Mpox virus?
According to the High Authority of Healththe vaccination campaign against the Mpox virus concerns populations at high risk of exposure to the virus, namely:
- men who have sex with men (MSM) and transgender people reporting multiple sexual partners;
- people in prostitution;
- professionals in sexual meeting places, whatever the status of these places
- partners or people sharing the same living space as the people mentioned above.
Primary vaccination with the MVA-BN vaccine (Imvanex or Jynneos) consists of the injection of two doses spaced at least 28 days apart, or a single dose for people who have received vaccination against smallpox with a first generation vaccine before 1980.