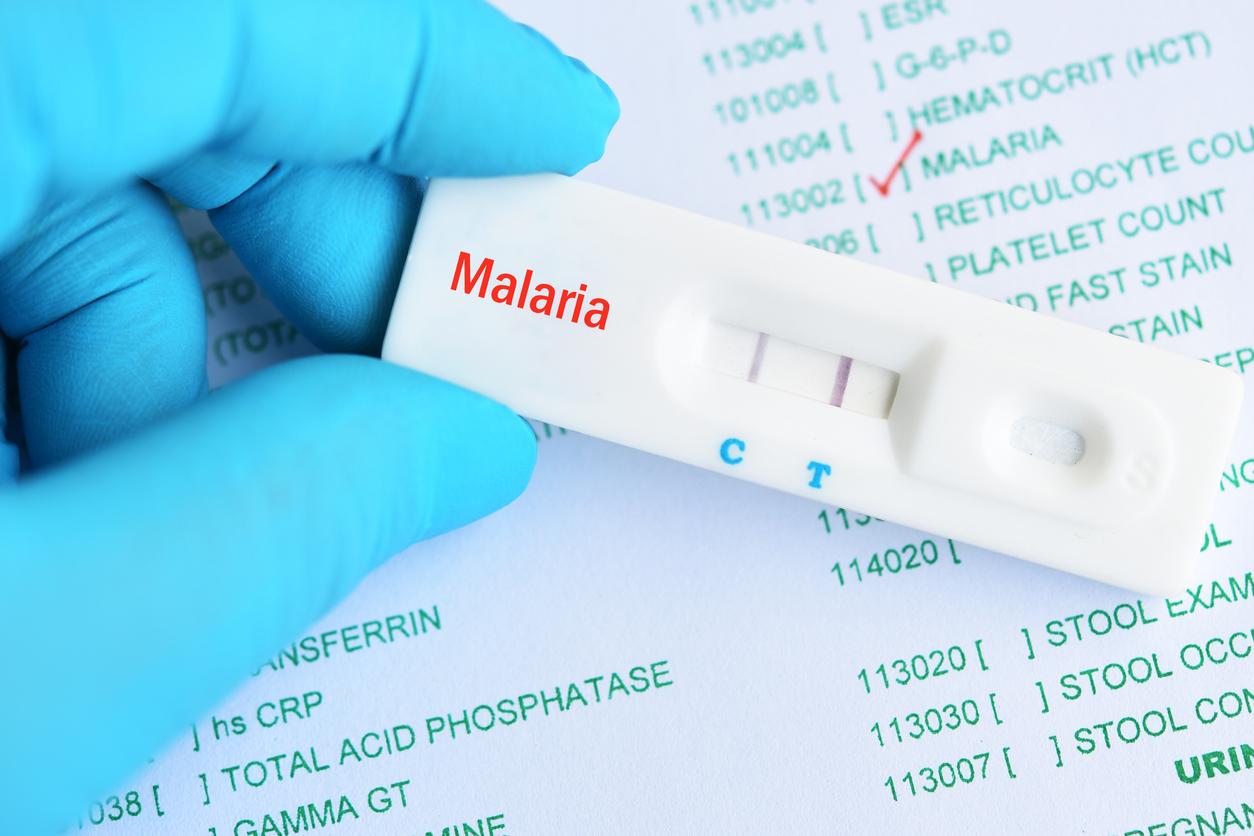
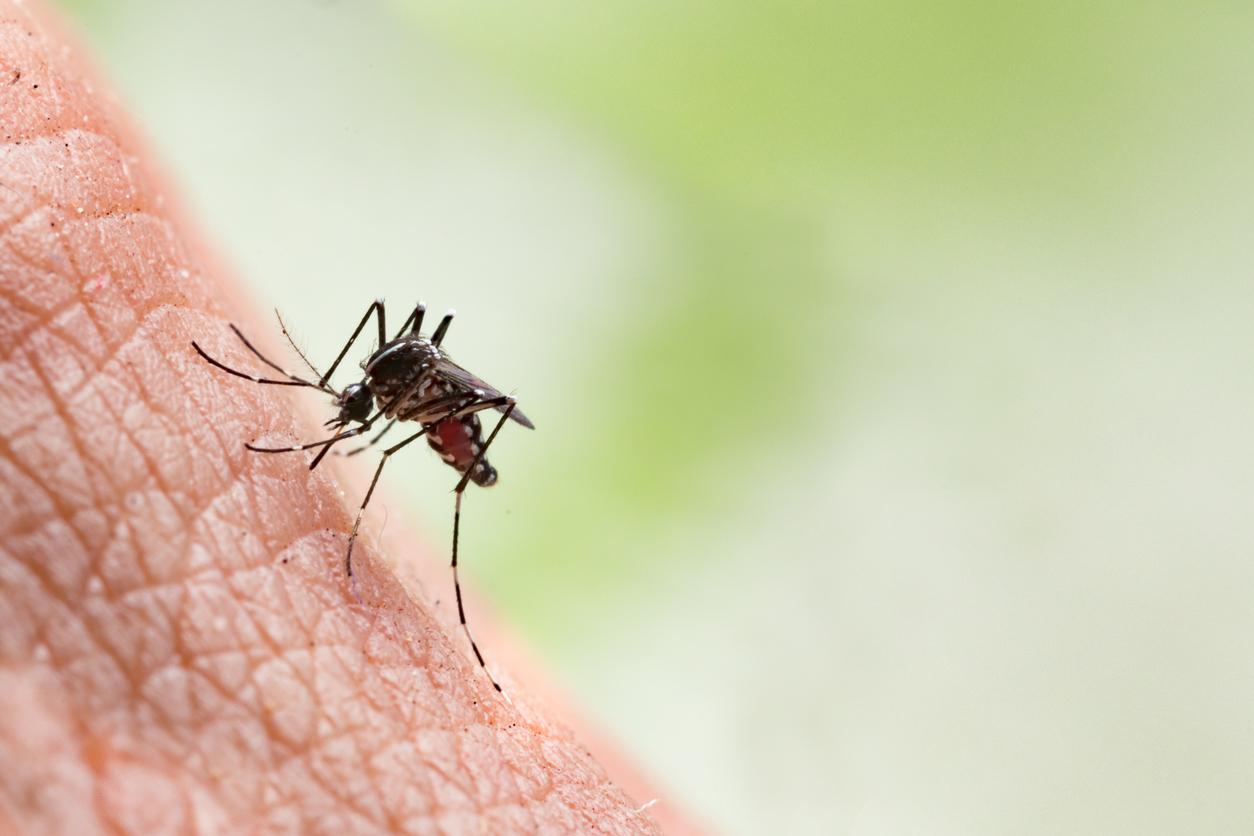
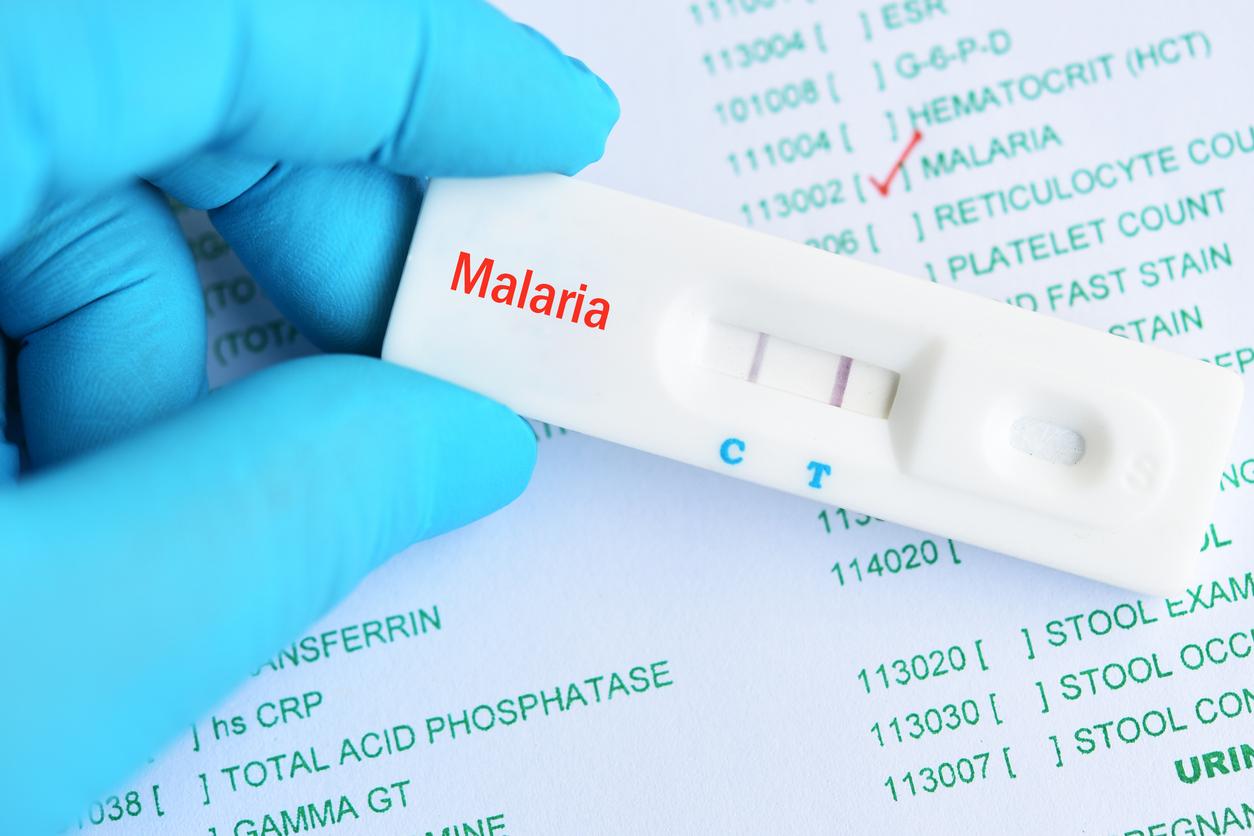
Malaria kills more than 700,000 people each year. A British laboratory is developing a vaccine. The results of the phase III study are hopeful.

The British laboratory GlaxoSmithKline (GSK) will apply for approval of its malaria vaccine in 2014. It has been developed with the organization for 30 years Path Malaria Vaccine Initiative (MVI). On the occasion of the publication of the results of its phase III study, this Tuesday, October 8, the pharmaceutical company has announced that it wants to market the RTS.S from next year.
Half the number of cases of malaria in children
The GSK laboratory conducted a clinical trial on 15,500 children in 7 different countries. This large-scale study raises hopes for a new preventive solution against malaria. 18 months after vaccination, RTS.S reduces the number of clinical malaria cases in children aged 5 to 17 months by 46% upon vaccination. It reduced more than a quarter of cases in children 6 to 12 weeks old. These populations are the most affected by the parasitic disease. Severe cases have also been reduced by a third and hospitalizations almost by half. The results of the study were analyzed taking into account the already existing preventive measures. This positive assessment inspires MVI.
RTS.S is a vaccine that activates the immune system in the presence of the parasite responsible for malaria, the plasmodium falciparum. It is believed to prevent infection, the development of the parasite and its invasion of the liver. In the phase III study, 3 doses were administered to each child, with an interval of one month between each injection.
A new weapon against malaria?
Malaria is a parasitic disease transmitted by mosquitoes. It kills 660,000 people every year. The victims are mainly children from sub-Saharan Africa. Curative treatments are often too expensive for affected populations. The spread of mosquito nets and other preventive measures have reduced the incidence of malaria. However, more efficient control tools are needed to eradicate the disease. In a press release, the GSK laboratory expresses its hope that theWorld Health Organization recommends the use of RTS.S from 2015.
Enthusiasm should be moderated: the results of the study have generally declined. The laboratory had published results of the mid-stage study. They then evoked a fall of 56% of clinical cases of malaria and of 47% of severe cases, that is to say a systematic fall of 10 and 20 points.
.