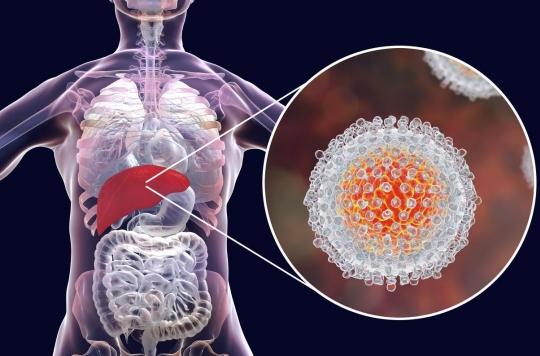
According to the Haute Autorité de Santé, the improvement in the actual benefit of the new treatment for hepatitis C, Harvoni, is minor compared to other drug combinations on the market.

More expensive, but not necessarily more efficient. The Gilead laboratory has announced the arrival of a new treatment for hepatitis C. Harvoni combines two antivirals, sofosbuvir – which already makes up Sovaldi – and ledipasvir. It costs 46,000 euros for a 12-week treatment. According to Gilead, this additional cost compared to Sovaldi alone is explained by the gain in efficiency. But the new drug brings “minor therapeutic progress” according to an opinion of the Transparency Commission of the High Authority of Health (HAS), posted on June 26.
Gilead reports treatment effectiveness of up to 99%, depending on the type of hepatitis C being treated (1, 3 or 4). Harvoni would therefore be more effective than Sovaldi alone. But it is also more expensive by 5,000 euros. The laboratory specifies that Harvoni could however make it possible to dispense with other drugs often associated with current treatments.
1. The Transparency Committee considered, in its opinion of 04 March 2015, that the proprietary product HARVONI provides a level IV (minor) improvement (ASMR) compared to the other sofosbuvir-based combinations currently available (sofosbuvir + daclatasvir and sofosbuvir + simeprevir), in the management of adult patients infected with HCV genotypes 1, 3 and 4, taking into account the following:
– its significant virological efficacy, similar to that observed with the other sofosbuvir-based combinations available (sofosbuvir + daclatasvir and sofosbuvir + simeprevir) but with a better level of evidence
– its satisfactory tolerance, resistance and drug interaction profile.
2. The costs concerning the various specialties associated with sofosbuvir were published in the Official Journal, and the cost of treatment with HARVONI (46,000 € for 12 weeks) is less expensive than that combining SOVALDI® with DAKLINZA® (66,500 € for 12 weeks) or SOVALDI® at OLYSIO® (€ 62,000 for 12 weeks).
The Transparency Commission recognizes the effectiveness of the treatment, especially at an early stage. But Harvoni “provides minor therapeutic progress compared to other sofosbuvir-based combinations currently available in the management of chronic hepatitis C genotypes 1, 3 and 4.” But the other combinations (sofosbuvir / daclastavir, sofosbuvir / simeprevir) are equally effective and safer. Risks of resistance to the new drug are also to be feared, according to HAS experts : “Data from in vitro studies and clinical studies show a strong genetic barrier for sofosbuvir and a weak genetic barrier for ledipasvir,” they explain.
.









-1590362612.jpg)