The High Authority for Health authorized self-tests and antigen tests for children under 15 on Monday. The objective: to break the chains of contamination at school.
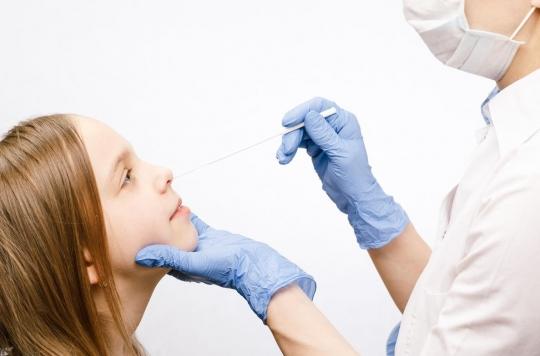
- The objective is to carry out 400,000 screenings per week by mid-May.
- High school and college students will be able to carry out the self-tests themselves.
- Saliva self-tests are not recommended due to lack of data.
While schools have been open again since Monday, thea High Health Authority (HAS) lifts the age limit for the use of self-tests and antigenic tests, then set at 15 years. The goal is to achieve 400,000 screenings per week by mid-May with the aim of “break them contamination chains” at school. Classes will close as soon as a positive case for SARS-CoV-2 is identified.
Less invasive tests adapted to the youngest
These new recommendations are based on “recent modeling work” carried out by the HAS according to which “these tests can be a screening tool for children under 15”, she wrote. The speed of the result, obtained in less than 30 minutes, and the “possible repeated use among a wider audience”, justifies their deployment among the youngest.
These tests have the advantage of being more suitable for the youngest. The saliva test is less invasive for children than nasopharyngeal swabs and the self-test requires pushing the swab less deeply into the nostrils than when performing PCR tests. HAS recommends that these tests be “carried out at least once a week according to the sampling methods best suited to the child’s age, abilities and local context.“High school and college students will be able to carry out the self-tests themselves. For primary school students,initially supervised self-sampling is (…) possible but it is preferable that the test be done by parents or trained personnel.“For children in kindergarten, “the sampling and the test must be carried out by these same actors.”
Saliva self-tests not recommended
The HAS has however expressed reservations about the use of rapid saliva tests. These self-tests should not be confused with conventional saliva tests, the results of which are analyzed in the laboratory. The authority advances a lack of data which “very heterogeneous, do not allow at this stage to show sufficient efficacy to be able to be recommended.”

.