An antidepressant drug, vortioxetine, has been shown to shrink tumors in more than 65 percent of glioblastoma patients.
- Scientists have discovered that an antidepressant can shrink tumors in glioblastoma patients.
- In France, a box of 28 tablets costs 44.64 euros.
- After research on cancer tissue and mice, scientists are set to test the antidepressant on patients.
In France, around 3,500 new cases of glioblastoma are diagnosed each year, according to University of Angers. This cancer, which affects the brain or spinal cord, is very aggressive. Despite treatments and surgery, there are often recurrences.
Antidepressant drug to treat glioblastoma
The results of a new study, published in the journal Nature medicinecould well change the management of glioblastoma. Researchers have discovered that an antidepressant drug could be very effective in treating this cancer.
“The advantage of vortioxetine is that it is safe and very cost-effective.“, says Dr. Michael Weller, co-author of the study, Daily Mail. According to the media, this drug is sold for around £40 in the United Kingdom, or a little over 47 euros, for a pack of 28 tablets. In France, the prices are similar: excluding dispensing fees, a box of 28 tablets is sold for 44.64 euros, according to the Vidal.
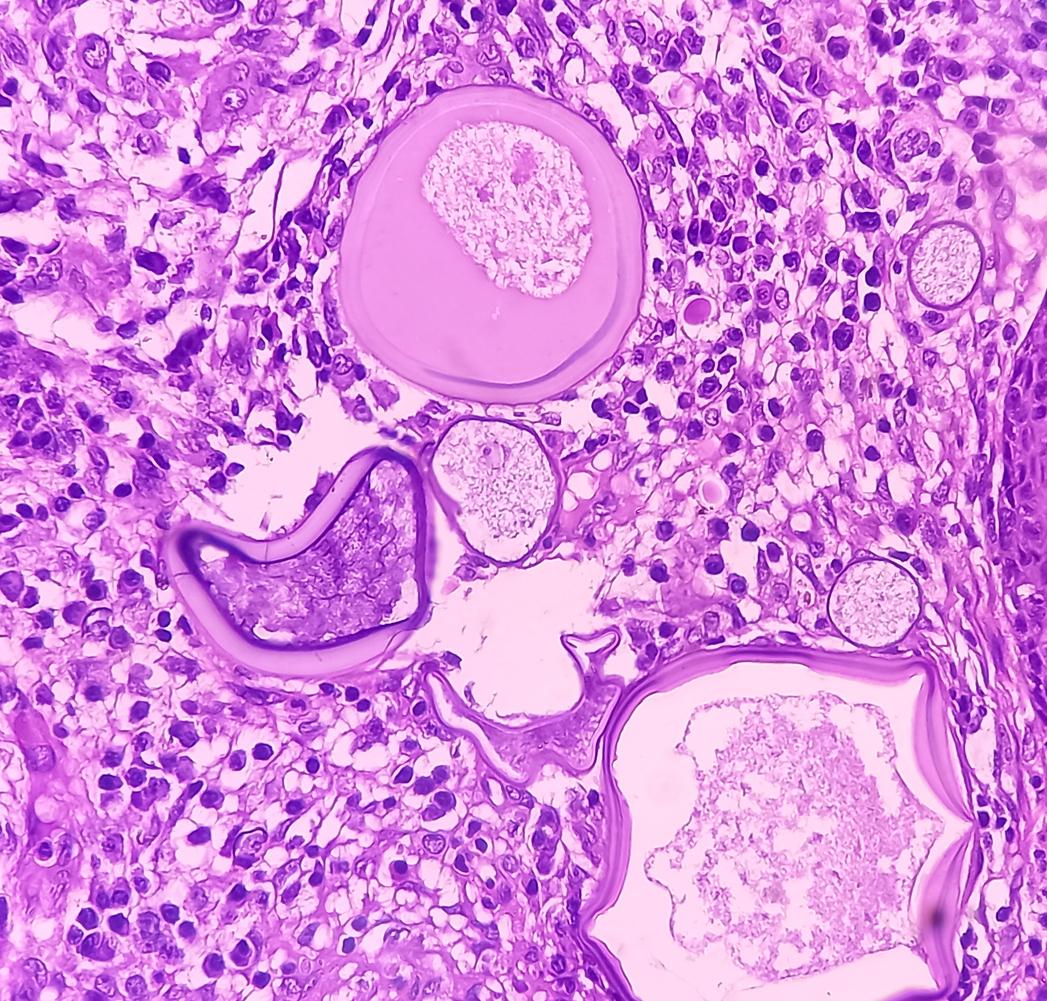
To find this drug, the researchers tested more than 130! Exactly 132 that they tried on cancerous tissues from 40 patients with glioblastoma. These patients had already undergone surgery for their cancer. At the same time, the scientists also conducted studies on laboratory mice.
Result: The antidepressant vortioxetine scored the highest. With this treatment, in 66.7% of patients, there was a decrease in tumors. In mice, scientists observed a reduction in tumors and a slowdown in their growth, especially if the antidepressant was taken in combination with chemotherapy.
Do not test it alone, before the drug is put on the market
“Since the drug has already been approved, it does not need to go through a complex approval process.“, points out Dr. Michael Weller. This means it could be brought to market more quickly than a new drug.
Before that, scientists still need to prove its effectiveness. The next step is to test this drug on humans. In the meantime, it is strongly advised not to test it alone in case of glioblastoma.
“We do not yet know whether the drug works in humans and what dose is needed to fight the tumor, so clinical trials are needed.”, concludes Dr. Michael Weller.