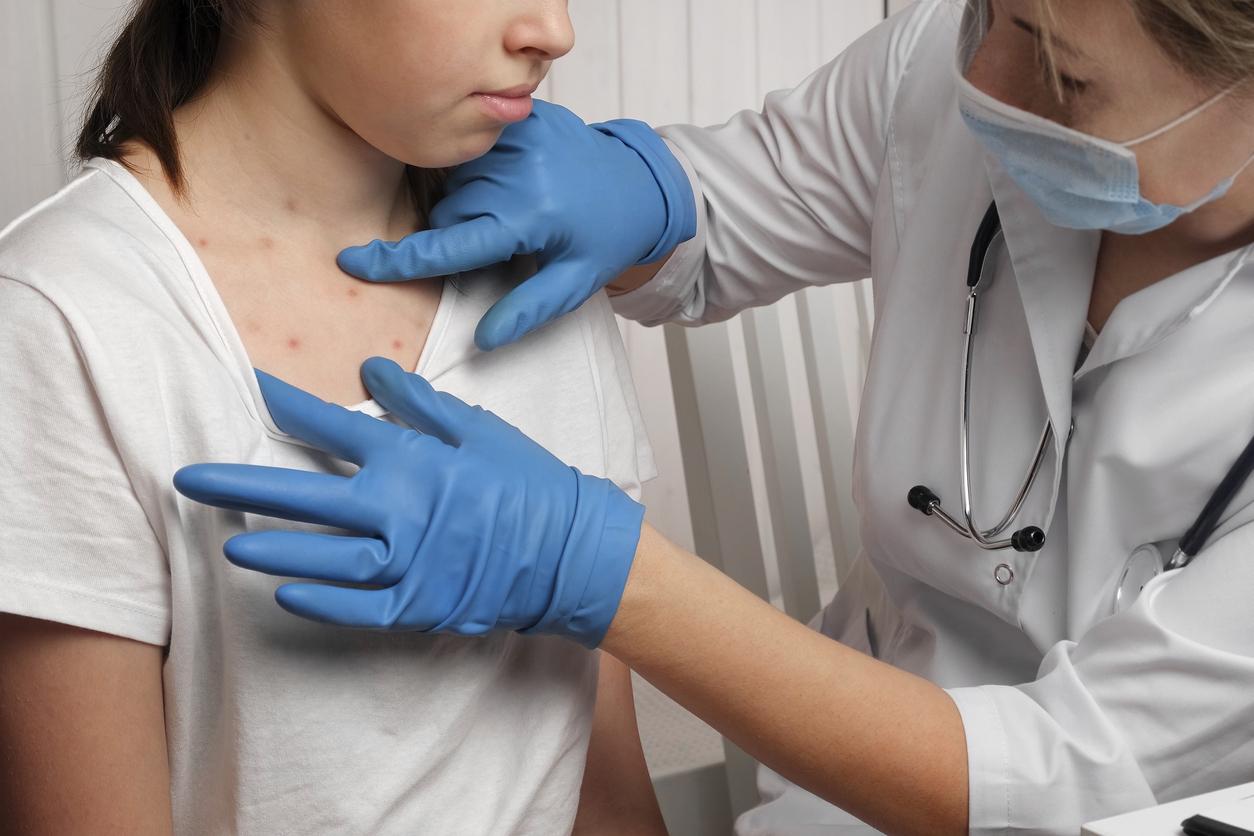
THE’Ebola outbreak in West Africa continues. Meanwhile, research is underway to find the most appropriate way to overcome this hemorrhagic fever which has already claimed 1,427 deaths.
The latest announcement concerning the sending of experimental treatments, therefore not approved (there is to date no scientifically proven vaccine to cure the virus) comes from the Japanese authorities. They stand ready to send an experimental drug manufactured by a Japanese company. “Our country is ready to deliver the drug in cooperation with the manufacturer if the World Health Organization (WHO) requests it,” said the secretary general of the government, Yoshihide Suga, reported by AFP.
Favipiravir (or “T-705”), that’s its name, is marketed under the name Avigan by Toyama Chemical, a subsidiary of imaging specialist FujiFilm Holdings. Unlike the Zmapp, the experimental serumSent to Liberia by the United States and which treated two American doctors last week, but failed to save a Spanish missionary, the Avigan was approved and licensed in Japan as an antiviral against the flu. He is in clinical trials in the United States. The treatment could be administered in the form of tablets, facilitating its distribution, especially in hard-to-reach areas in countries affected by Ebola (Sierra Leone, Guinea, Liberia, Nigeria and now the Democratic Republic of the Congo).
Japan says it has enough stock to help 20,000 people. The Zmapp is out of stock since it had been produced in limited quantities.