Isotretinoin, which is well tolerated, has led to an improvement, or even disappearance, of acne in transgender people undergoing masculinizing transition.

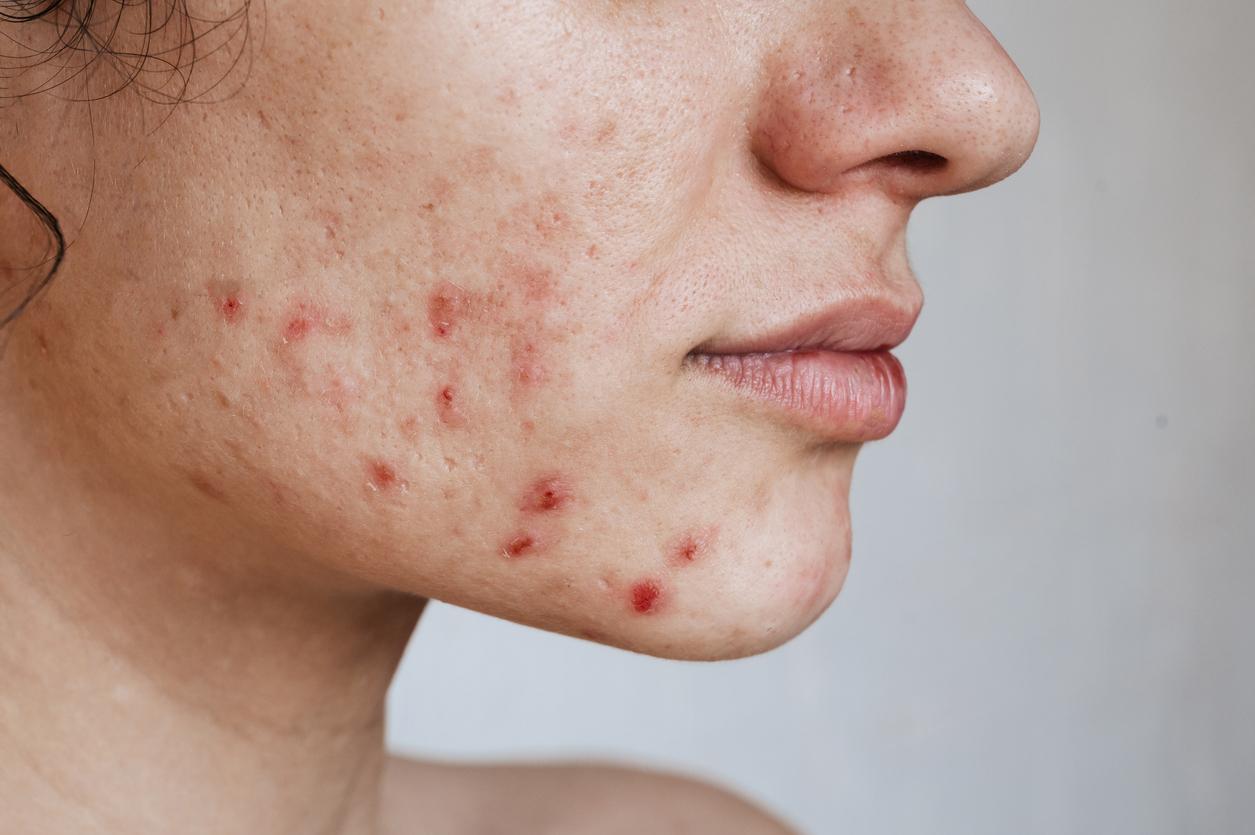
- Masculinizing hormone treatment, that is, treatment aimed at reducing secondary sexual characteristics of the unwanted gender, promotes the development of acne.
- Isotretinoin is associated with improvement in 97% of transgender individuals and resolution in 63.6% of patients treated with a cumulative dose of 120 mg/kg or more.
- Dryness, joint pain and eczema were the most commonly reported side effects.
As part of the transition process for transgender people, hormonal treatment aims to attenuate the secondary sexual characteristics of the unwanted gender (physical characteristics, apart from the sexual organs, which differentiate the two sexes: more developed breasts in women, greater body hair and muscle mass in men, etc.) and to promote the development of those of the desired gender. According to Insermthis is done through a combination of hormones that lowers the level of sex hormones naturally produced by the person.
Acne: 55 people receiving masculinizing hormone therapy received isotretinoin
“Followed over the very long term, sometimes several decades, this treatment can, like any medication, induce undesirable effects.” Among these is acne, according to researchers at Brigham and Women’s Hospital in Boston, United States. In a recent study, published in the journal JAMA Dermatologythey wanted to know if isotretinoin, an effective acne treatment, held promise for transgender people transitioning. To find out, they recruited 55 people aged 12 to 49 who were receiving masculinizing hormone therapy. Between August 14, 2015, and September 20, 2023, they were prescribed isotretinoin to treat their acne. The median duration of treatment was six months, with a median cumulative dose of 132.7 mg/kg. The median cumulative dose was less than 90 mg/kg for 16 participants and less than 120 mg/kg for 22 volunteers.

Acne clears in 47% of transgender people
According to the results, the drug was associated with improvement in 87.3% of patients and clearance in 47.3% of volunteers. For the 33 participants treated with a cumulative dose of 120 mg/kg or more, these rates increased to 97% and 63.6%. Among the 20 patients who achieved clearance of acne and subsequently consulted a doctor, the risk of recurrence was 20%. The most commonly reported side effects were dryness, joint pain and eczema. “Cost, pharmacy issues, side effects, logistical reasons and wound healing issues for gender affirmation surgery were the reasons for premature discontinuation of treatment,” can be read in the research.
In the conclusions, the authors indicate that further efforts are needed to understand optimal dosing and barriers to treatment in order to improve outcomes in transgender people receiving masculinizing hormone therapy.