The manufacturers intend to participate in the return to confidence in the drug in the face of a climate of suspicion. The new president of LEEM denounced the hypocrisy of the public authorities.

In France, “each question about a drug turns into an indictment,” regretted yesterday Hervé Gisserot, president since last December of Leem, the body which brings together drug companies.
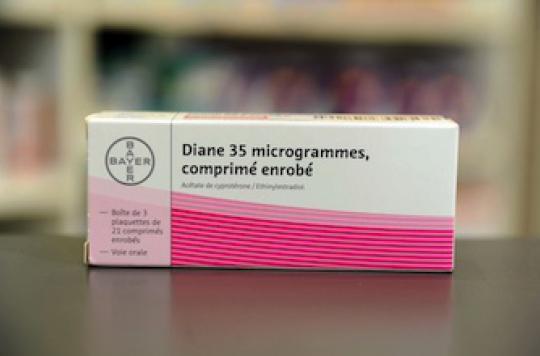
Stressing that he was not the spokesperson for Bayer, laboratory manufacturer of Diane 35, Hervé Gisserot regretted that the announcement of the 4 deaths linked to the taking of this drug as a contraceptive was not “put into perspective “with the useful life of 25 years. He also specified that like any health product, Diane 35 is the result of a fragile balance in its benefit / risk balance. More generally, the boss of Leem reiterated that the risk was “intrinsic to the nature of the drugs”. “Our companies must continue to mobilize around the detection of adverse effects,” he said. In addition, he underlined that half of the negative effects reported by pharmacovigilance were already reported by manufacturers, while pleading for the development of pharmaco-epidemiology with the request for the opening of public health databases.
But his most severe charge, it is to the public authorities that he reserved it. “The State claims to revive France’s industrial policy,” he attacks, “but at the same time it is organizing the contraction of the market. He wants to encourage the scientific influence of France, but he bans medical congresses by a totally dissuasive tax system. “. According to him, “France has let itself be taken down”, he assures, “by dint of deploying purely Franco-French provisions which result in losses of competitiveness”. The number of marketing authorization files for which France is the rapporteur is an illustration of this. While the Hexagon was located a few years ago just behind the United Kingdom, “it is today at the level of Malta or Cyprus”, he concludes.
.