The death of the 5th CARMAT patient is linked to the interruption of the power supply to his artificial heart, following poor handling of the batteries by the patient.
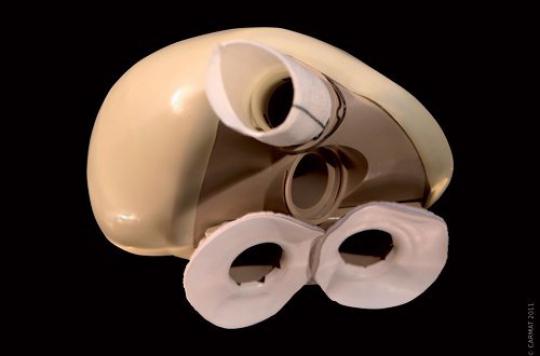
We know more about the death of the fifth patient implanted by an artificial heart from the CARMAT company. As indicated in its press release of November 30, 2016, the company now confirms that the prosthesis functioned correctly during its use by the 1st patient of the PIVOT study, the second phase of the ongoing clinical trial. Four patients had been implanted previously.
But CARMAT does not stop there. The company judges this Tuesday said Tuesday that “the death is linked to the interruption of the power supply to the system, following improper handling of the batteries by the patient which caused the stopping of the prosthesis”.
The suspended clinical trial
She specifies on this subject that the Support-Training teams of CARMAT are working “actively on this aspect relating to post-operative follow-up in order to strengthen the safety of future patients”.
Because the test is still at a standstill, we learned on Monday. In the columns of Parisian, Carmat’s boss, Stéphane Piat, was even impatient to still wait for the green light from the National Medicines Safety Agency (ANSM) to restart the establishments.
But for the moment, CARMAT recognizes that it is not in a position to meet the requirements required by the ANSM within the allotted time. He nevertheless plans to submit “a new request shortly with the required elements”. “The conduct of the PIVOT study in France will remain suspended until the acceptance of this new request by the ANSM”, she adds. Perhaps the time to leave it to Carmat to guarantee the safety of the replacement of the batteries of his artificial heart.
A departure in the United States possible
But Carmat does not hesitate to praise once again the qualities of its technological prowess: “The prosthesis has functioned normally during the last 3 implantations (…) Our confidence is all the stronger than the results provided by the self-regulating system used during the last installation were very encouraging, ”concludes the CEO of CARMAT.
The latter, however, leaves the threat of settling abroad. “We are ready to go. But I wonder, replied Stéphane Piat to Parisian. Do we really want to innovate in France? »The United States, for example? The FDA, the US health authority, he admitted, has “a more pragmatic approach.” One thing is certain, however, even if the trial restarts in France, the company will refuse to continue at the rate of two trials per year.
.

















