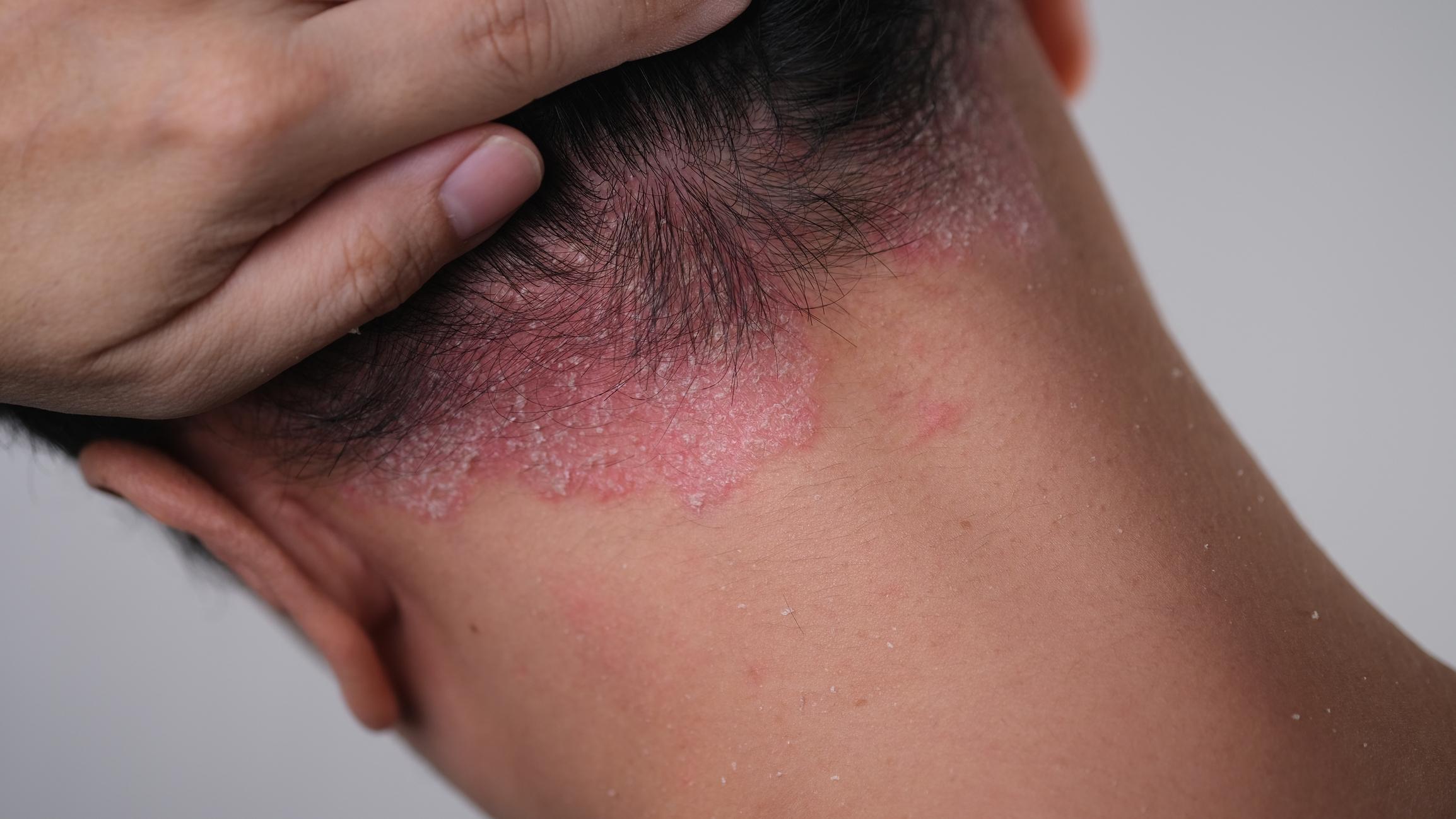
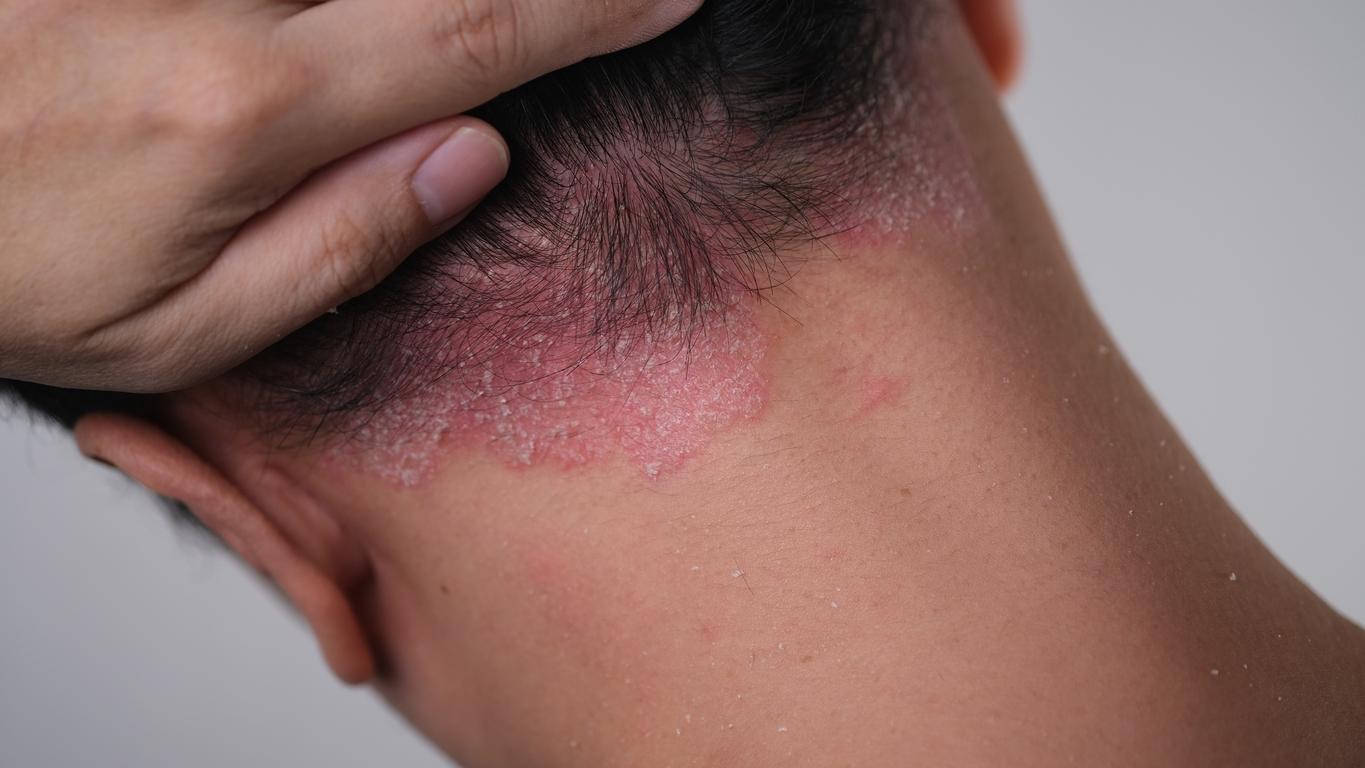
Efaluzimab (Raptiva) has been marketed in France since 2005. It is indicated for the treatment of adult patients with moderate to severe chronic (long-term) plaque psoriasis. Dermatologists use it in patients who have not responded to other systemic treatments (which spread throughout the body) for psoriasis or who are intolerant to these types of treatments, such as ciclosporin, methotrexate and PUVAtherapy (psoralen and ultraviolet A).
The Medicines Evaluation Agency (EMEA) revised its marketing authorization at the request of the European Commission, which was alerted by the occurrence of 3 cases of progressive multifocal leukoencephalopathy (PML), in patients with took the Raptiva for over 3 years. Progressive Multifocal Leukoencephalopathy (PML) is a serious, progressive brain infection that can lead to death or severe disability. As a result of these cases, Emea concluded that the risk-benefit ratio of efaluzmab had become unfavorable.
To date, the French Agency for the Safety of Health Products (Affsaps) estimates that 560 patients are being treated. She recommends that they do not suddenly stop this treatment but to consult their prescribing doctor quickly in order to set up a replacement treatment.
Find out more about the disease: Treating psoriasis well