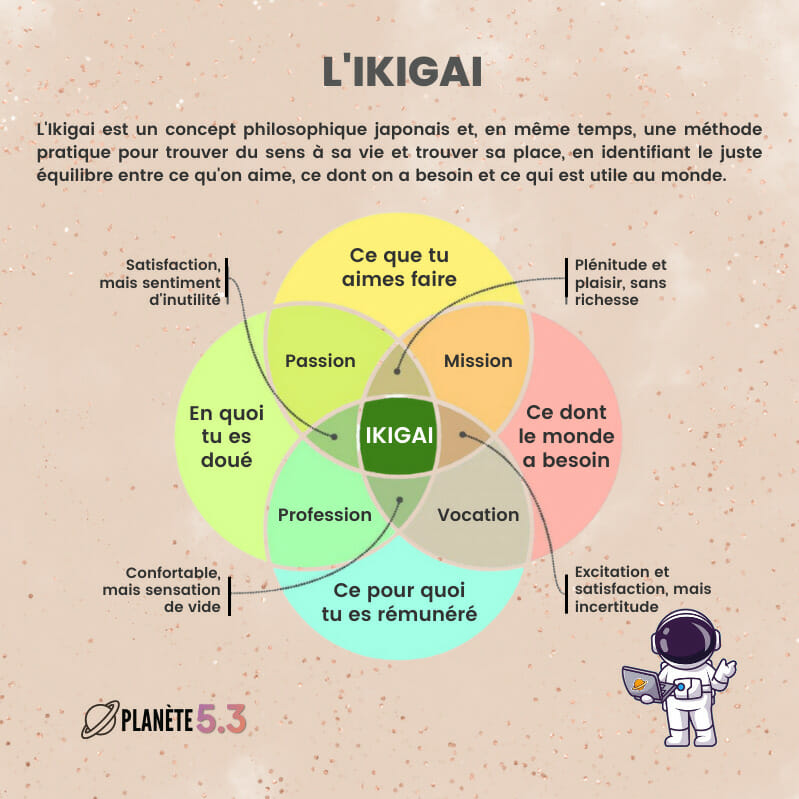
The European Medicines Agency (EMA) announced in a press release the launch of the evaluation of the Pfizer / BioNtech vaccine in 12-15 year olds.
A European vaccine for adolescents
While the American company Pfizer and the German company BioNtech have both already applied for marketing authorization for their vaccine for adolescents, evaluation of the latter began on May 3. The EMA is due to deliver its opinion during the month of June. As the Agency states, the Medicines Committee “ will carry out an expedited assessment of the data submitted by the company that markets [ce vaccin], including the results of a large ongoing clinical study involving adolescents from 12 years of age, in order to decide whether or not to recommend »The extension of this vaccine to the youngest.
The use of the Comirnaty vaccine from Pfizer and BioNtech could then extend to 12-15 year olds and begin by this summer. However, each member country will be free to expand vaccination against Covid-19, depending on its vaccination strategy.
The clinical study for the Pfizer / BioNtech vaccine included 2,260 American adolescents. The efficiency would be optimal, as BioNtech indicated: “ The first results we have seen in studies of adolescents suggest [qu’ils] are particularly well protected by vaccination “.