By explaining the mechanism of oxygen regulation in cells, the 2019 Nobel Laureates in Medicine paved the way for the development of new drugs against anemia and, above all, against the proliferation of cancer cells.
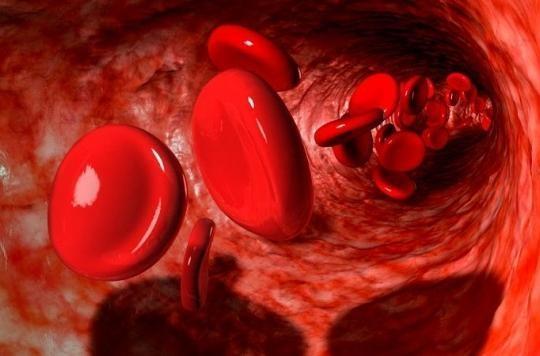
Two Americans and a Briton were honored by the Swedish jury for their work on how cells adapt to the availability of oxygen. These discoveries are fundamental and offer perspectives in the treatment of many pathologies. The work of these three researchers, Willialm Kaelin, Gregg Semenza and Sir Peter Ratcliffe, whose publications have earned them distinction today, dates from the years 1990-2000. These have made it possible to understand how the organism adapts to variations in the availability of oxygen. More specifically, they solved the mystery of the molecular mechanism behind adaptation to hypoxia.
We have known for centuries that oxygen is essential for the functioning of the human body. By contrast, how our bodies adapt to variations in this much-needed molecule was still a mystery until the work of the three Nobel laureates in medicine through which we know more about how different oxygen levels regulate basic physiological processes.
At high altitude, oxygen is lower than elsewhere. In response, cells will release doses of erythropoietin (EPO), a hormone – well known to “athletes” since it is the one that has been used in cases of doping – which leads to an increase in the production of erythrocytes , red blood cells, which allows the body to adapt to the regular lack of oxygen. The body is thus able, at the cellular level, to adapt to a change in oxygenation. Apart from the environment, the body itself can weaken its oxygen supply, for example in the event of physical exercise but also in certain pathologies.
Hypoxia-induced factors bind to identified DNA segments
At the heart of the discovery of the rewarded scientists, the transcription factors HIF (hypoxia inducible factors, or factors induced by hypoxia). A “transcription factor” is a protein that regulates the transcription of a gene. In this case, the HIF acts on the genetic expression of the tissues in the event of a drop or lack of oxygen.
As we said for the high altitude environment, a situation of hypoxia (lack or absence of oxygen) generates the increase in EPO. By experimenting on genetically modified mice, Gregg Semenza discovered specific DNA segments linked to EPO and which serve as mediators in responding to hypoxia. He realized that the regulation of oxygen in all the cells of the body was done thanks to two very special proteins, “HIF-1α” and “ARNT”, as well as a gene called VHL. They come to bind to these identified DNA segments. It is this set of proteins that he called “hypoxia-induced factors” (HIF). When cells lack oxygen, the number of HIF-1α proteins increases and influences a hormone, the famous EPO. This hormone notably helps to control the production of red blood cells, the blood cells that carry oxygen.
Discoveries essential to combat certain diseases
We also know, thanks to their research, that the von Hippel-Lindau (VHL) gene has an important impact in all this regulation. Thus, this gene is an important lead in the face of certain diseases that involve the overproduction or underproduction of red blood cells. For example, patients with chronic renal failure often suffer from severe anemia due to decreased expression of EPO. EPO is produced by kidney cells and is essential to control the formation of red blood cells, as explained above. Also, the oxygen regulatory mechanism has an important role in cancer. In the case of tumours, for example, oxygen is regulated in such a way as to remodel the metabolism and facilitate the proliferation of cancer cells.
University laboratories and pharmaceutical companies are therefore trying to develop “drugs from interfering at different stages of a pathology”explains the Nobel Prize jury: either by “activating”either “blocking the oxygen uptake mechanism”.