The authorizations of 9 generic drugs are suspended. The ANSM has serious reservations about the integrity of the data from bioequivalence tests carried out in India.

The National Medicines Safety Agency (ANSM) is on the lookout. In a press release published on Tuesday, it announced that it had suspended the marketing authorizations (AMM) of 9 specialties marketed in France, in particular antimalarials. This “pending the outcome of the European arbitration procedure,” she said.
But these suspension measures, which took effect yesterday, are taken “as a precaution”, she reassures. It indicates that to date, nothing has led to the establishment of a proven risk for human health or a lack of effectiveness of these drugs. She adds that “these suspensions concern only specialties containing 3 active substances (or combination of active substances) and no risk of rupture or interruption of treatment is to be feared insofar as these drugs are available under other drugs. brands ”.
Manipulated test results
To understand the publication of this decision, the ANSM recalls that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has, at its meeting in April 2016, initiated a procedure to re-evaluate the benefit / risk ratio of the specialties for which the clinical studies were carried out in the private Semler Research center located in Bangalore, India.
This review follows inspections conducted by the Food and Drug Administration (FDA) of the United States of America and the World Health Organization (WHO), which revealed manipulations of the results ofbioequivalence assays (1) by replacing biological samples from certain subjects with samples from other subjects. “These manipulations characterize a lack of respect for Good Clinical Practices concerning these bioequivalence studies and call into question the reliability of the results of all the bioequivalence tests conducted by this company”, indicates the ANSM.
Several recalled lots
Other Member States have also taken national measures to suspend marketing authorizations for the medicinal products concerned present on their market. This as provided for by European regulations, pending the final decision of the European Commission.
“The batches of these products present in pharmacies, health establishments and wholesale distributors are subject to a recall (see the list below)”, concludes the ANSM.
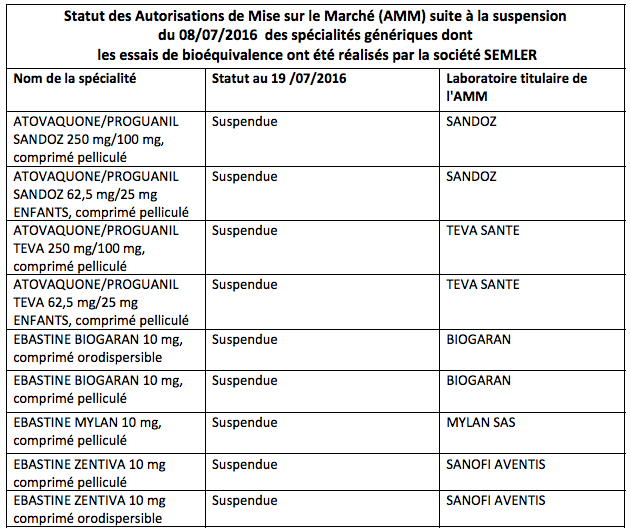
Source: ANSM
.

















