The Medicines Safety Agency publishes a brochure on the pill for patients. It details the different types of contraceptives and the possible risks.
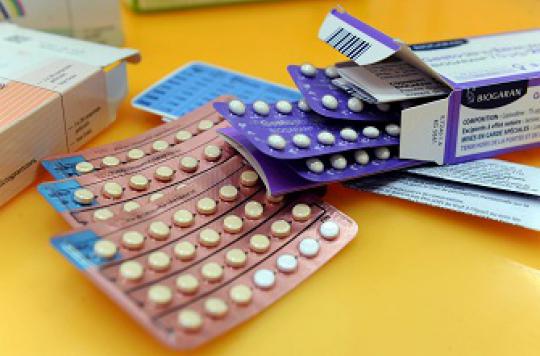
How to assess the risks of contraception? To reassure women, the Medicines Safety Agency (ANSM), in association with the Haute Autorité de Santé (HAS), is publishing a brochure on estrogen-progestogen pills on January 10. The aim is to help women learn more about the contraceptive they use the most and to better identify its risks.
The ANSM brochure will be distributed to healthcare professionals, who can give it to their patients. She is also downloadable from the agency’s website. Contraceptive use, precautions and potential side effects are detailed there. The ANSM takes this opportunity to indicate the procedure to follow in the event of thrombosis or even a heart attack.
In January 2013, the Agency began a reassessment of the benefit / risk ratio of combined hormonal contraceptives (pill, vaginal ring, patch) containing 3rd and 4th generation progestins. It is now recommended to avoid these treatments as much as possible and to assess the patient’s risk before prescribing them. In November, the European Medicines Agency (EMA) maintained a favorable report, while recalling the risk – rare but serious – of venous thromboembolism in patients at risk.
.